At the Heart of the AtomIntroduction |
How is radioactive decay used to determine dates? |
Radioactive carbon-14 (14C) has a half life of 5,730 years. It is produced in the atmosphere when cosmic ray neutrons strike nitrogen atoms. The 14C then reacts with oxygen to form carbon dioxide in the atmosphere and dissolves into the oceans. Plants take up the atmospheric CO2 in respiration and animals ingest it. Therefore all living things exchange both 12CO2 and radioactive 14CO2 throughout their lives. When they die the exchange stops and the fraction of 14C to 12C in their bodies is fixed. As time goes on the amount of 14C decreases. Objects up to 50,000 years old can be dated by radioactive carbon as long as correction figures, agreed to by international agencies, are applied. Willard F. Libby (1908-1980), an American chemist, won the Nobel Prize in chemistry in 1960 for his development of carbon-14 dating in 1949.
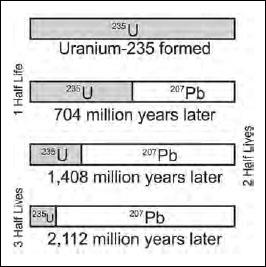
There are several other dating techniques that use the half lives of radioactive isotopes in rocks including uranium/thorium and rubidium/strontium ratios that extend dating techniques to hundreds of millions of years.
What happens to an element when it decays by emitting an alpha particle?
The nucleus that results from a radioactive decay is called the daughter. The number of nucleons in a radioactive decay does not change, and in an alpha decay the number of protons of the original must equal the number of protons in the daughter plus the number of protons in the alpha. The same is true for the number of neutrons. So, for example, when uranium-238, with 92 protons and 146 neutrons emits an alpha, the daughter has 90 protons and 144 neutrons. It is thorium-234. The alpha is emitted with a specific energy. Only very heavy nuclei decay by alpha decay.